// P01
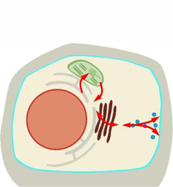
Lipid-cytoskeleton interactions during stress-induced ER-plasma membrane contacts
Project Leader// Prof. Dr. Ingo Heilmann
Martin Luther University Halle-Wittenberg
Institute of Biochemistry and Biotechnology
Plant Biochemistry
Salt and osmotic stress are important environmental cues for plant growth and result in changes in gene expression within minutes to hours of application. Physiologically, hyperosmotic stress results in an immediate (i.e., within few seconds) loss of turgor, requiring short-term adjustments of the plasma membrane, which represents the outermost barrier of the cell. Despite their importance, the mechanisms ensuring the integrity of the plant plasma membrane under osmotic stress are not well understood.
Recent evidence demonstrates that organellar membrane contact sites form in the model plant Arabidopsis thaliana upon salt-treatments, suggesting such contact sites may be part of the physiological stress response. The stress-induced formation of ER-plasma membrane contact sites involves the action of synaptotagmin 1 (SYT1) and its interaction with the regulatory membrane lipid, phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2). The analysis of PI(4,5)P2 dynamics by a fluorescent biosensor suggests transient stabilization of PI(4,5)P2 during salt stress conditions also facilitating the formation of ER-plasma membrane contacts. However, the role of PI(4,5)P2 in this process is currently unclear. One hypothesis is that PI(4,5)P2 is required to bind SYT1, and we additionally propose that the formation of ER-plasma membrane contacts also requires the localized association of the cytoskeleton. Recent work by P01 showed a novel PI(4,5)P2-binding feature of class VIII myosins, which is required for a stabilizing effect of class VIII myosins on actin dynamics. Moreover, PI(4,5)P2 nanodomains at the plasma membrane are involved in the stabilization of actin filaments. As class VIII myosins likely mediate actin-plasma membrane attachment, we hypothesize that these enigmatic proteins also contribute to the formation of ER-plasma membrane contacts and might, thus, be required for plasma membrane remodeling during the Arabidopsis salt response. It is the goal of ongoing work in project P01 to address how stress-induced ER-plasma membrane contact sites are formed, how actin filaments are contributing, and to determine whether either or both are required for changes in plasma membrane lipid composition and/or nanostructure upon stress. The experimental work employs the combination of a broad range of genetic, molecular, biochemical and cell-biological approaches.



